- Who were the first people to use the phrase 'atom'?
- The Greeks
- The Vikings
- The Chinese
- The Romans
- Which of these could be used as a fuel in a nuclear fission reactor?
- Uranium-235
- Tritium
- Deuterium
- Plutonium-239
- Look at the nuclear symbol for uranium 235.
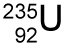
What does the number 92 stand for?- Atomic number
- Mass number
- Number of protons
- Number of neutrons
- The diagram shows an uranium atom splitting, what name is given to this process?
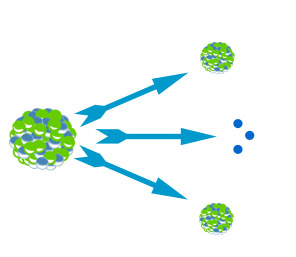
- Nuclear fusion
- Nuclear fission
- Nuclear fizzing
- Nuclear decay
- The diagram shows the plum pudding model of an atom. Which part of the atom do the plums refer to?
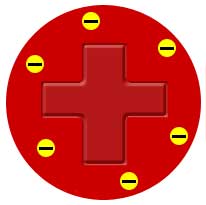
- Nucleus
- Protons
- Electrons
- Neutrons
- In the diagram of the nuclear reactor X points to the part that is lowered into and out of the reactor to control the rate of fission. What are these called?
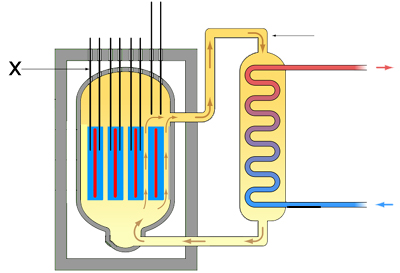
- Fuel rods
- Control rods
- Moderator
- Coolant
- The nuclear symbol for uranium-235 is. How many neutrons are present inside the uranium nucleus?
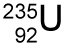
- 92
- 235
- 143
- 327
- The diagram shows three obseravtions made during Rutherford's alpha scattering experiment. Which observation provides evidence that the nucleus of an atom is very small?
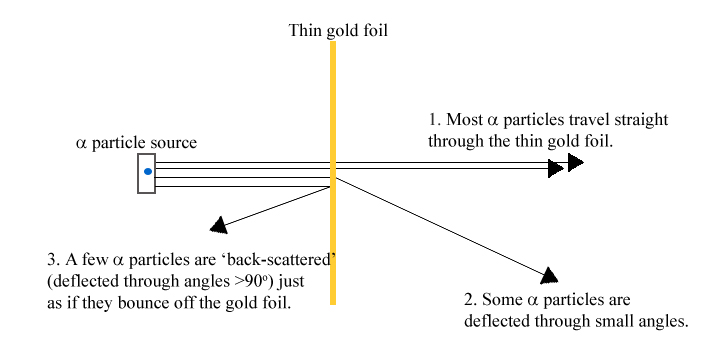
- Most alpha particles travel straight through the thin gold foil.
- Some alpha particles are deflected through small angles.
- A few alpha particles are ‘back-scattered’ just as if they bounce off the gold foil.
- The nuclear symbol for uranium-235 is. How many protons are present inside the uranium nucleus?
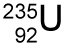
- 92
- 235
- 143
- 327
- What is the missing number in the nuclear equation?
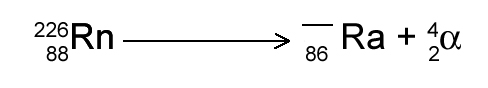
- 228
- 230
- 222
- 92
- In his atomic model how did Neils Bohr solve the dilemma of atomic spectra?
- By only allowing electrons to occupy certain orbits.
- By allowing electrons occupy any position around the nucleus.
- By putting all the positive charge in the nucleus.
- By assuming that the electrons float round in a positive fluid.
- What term is used to describe this process?
- Induced nuclear fission
- Spontaneous nuclear fission
- Nuclear fusion
- Nuclear fizzing
- What are beta particles?
- Fast moving electrons
- Neutrons
- A helium nucleus
- A helium atom
- What are alpha particles?
- Fast electrons
- Neutrons
- A helium nucleus
- A helium atom
- Which of these are isotopes?
- Where would we find the fuel required to power a nuclear fusion reactor?
- The sea
- The ground
- The air
- In space
- Which statement best describes nuclear fusion.
- Light atoms are fused together into heavier atoms.
- Heavy nuclei are split into smaller nuclei.
- Light nuclei are fused into heavier nuclei.
- Heavy nuclei are fused to form heavier nuclei.
- During nuclear fusion hydrogen is fused into which element?
- Helium
- Hydrogen
- Carbon
- Uranium
- What best describes this diagram?
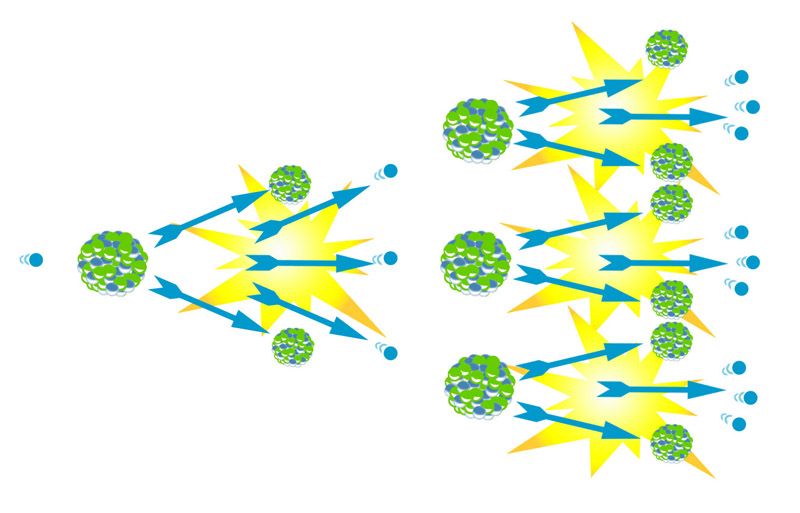
- Nuclear fusion
- Chain reaction
- Neutron capture
- Alpha decay
- We have not yet managed to make a nuclear fusion reactor. What two conditions are required to get nuclear fusion to work?